Clinicians often refer to patient reported outcomes (PROs) as patient surveys, quality of life measures, questionnaires, or waiting room activities. But the formal definition comes from the Food and Drug Administration (FDA) in the United States, which defines PROs as:
“…any report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else.”1
The reason we use the definition from the FDA stems from the history of PROs. PROs got its footing in clinical trials conducted in the mid-1990s, when health economists were trying to quantify subjective reports changes in study participants’ health conditions2. While PROs are still used in clinical trials, they have grown to be used in real-world clinical practice, including as means to track symptom history, monitor the effects of an intervention, and as an outcome measures for quality improvement or performance evaluation3.
The FDA definition is itself subject to some interpretation depending on the conceptual, operational, and administration models of interest. This post will focus on the various conceptual models that can be applied to defining and measuring PROs.
A Range of Conceptual Frameworks
The term “health condition” in the FDA definition can be interpreted through a spectrum of conceptual frameworks. As illustrated by figure 1, the spectrum is anchored by “quality of life” on one end. This would not only include health-related factors that have an impact on the quality of one’s life, but also broader factors such as work productivity and social engagement. In their book, Fayers and Machin adopt the World Health Organization’s mission in defining health condition through a quality-of-life lens4,5.
The broad conceptualization of health defined by the quality of life frameworks have several benefits. One of those is that they’re applicable to all patients – indeed, the respondent need not even be a patient – regardless of the ailment they’re dealing with or the treatment they’re receiving. This means clinicians can have one framework to work from, simplifying downstream data collection and analysis.
A second benefit of general quality of life frameworks is that they can be used to compare the health, or change in health, of patients in a general population. This might be useful for clinicians wanting to look at their entire patient panel, or compare the health of their patients to that of another clinician. Using this general conceptual definition means that we’re not restricted in our comparisons by narrowly defined concepts of health.
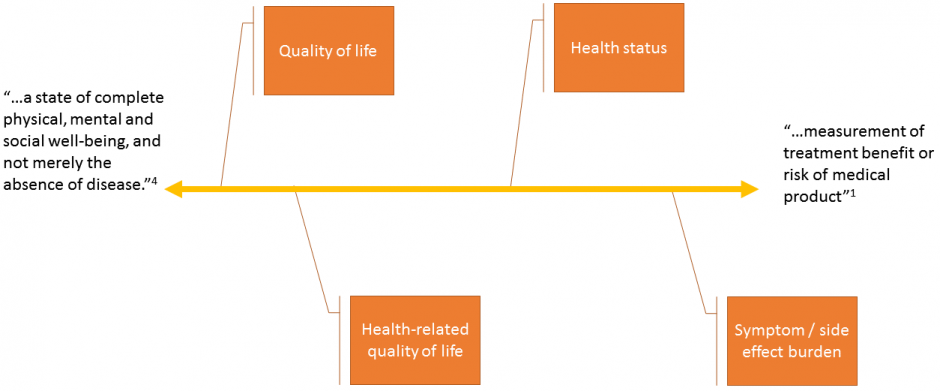
Figure 1. Spectrum of conceptual models for health condition.
On the other end of the spectrum is “side effect / symptom burden”. Through this concept, health is defined through factors that relate directly to a condition, organ system, or intervention. This narrow framework results in factors that are very specific, and likely unique, to a specific health condition4. For example, mobility and physical function of a hand might only be relevant to those suffering from osteoarthritis or rheumatoid arthritis being treated with medications, physiotherapy, or surgery.
The advantage of the narrowly conceptualized symptomatic framework is that they define health through the specific symptoms or functional limitations that clinicians are likely trying to treat. Therefore, the PRO instruments based on these frameworks can be used to inform medical decision making.
Another advantage to these specific frameworks is that the patient can relate more intimately with the health condition being measured. Thus, PRO instruments based on this conceptual framework tend to ask more relevant questions. As a result, they are more sensitive to changes in symptom severity.
Choosing the Framework to Meeting Your Needs
There are no right or wrong conceptual frameworks for defining health conditions, only different frameworks to meet different needs. If you are considering including a PRO instrument in clinical practice, you want to be sure that the conceptual framework it is based upon matches what you are wanting to measure and how you are intending to use those measurements.